MDR and UDI Labeling on Medical Devices Webinar
FOBA Laser Marking and Engraving is presenting a new webinar at 4pm on Tuesday 9th of June.
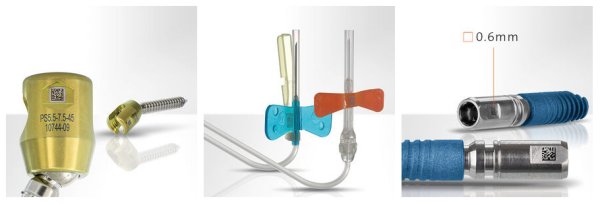
The webinar subject will be the UDI labeling on medical devices according to the latest requirements of the European Medical Device Regulation. The webinar will be presented in the form of an expert talk, followed by participant’s questions.
Your hosts Christian Söhner (FOBA Laser) and Michael Galliker (Regulatory Globe) will discuss:
how companies can approach the implementation of the UDI according to the MDR (keyword “gap assessment”)
the relevant MDR regulations for direct part marking
possible adjustments to the deadlines due to the latest MDR postponement
the technical options for a UDI direct marking
For more information go to the FOBA Website – www.fobalaser.com

Talk to Us!
Connect with our Technical & Support Team.